Executive playbook for scaling AI in life sciences


Table of contents
Subscribe via Email
Subscribe to our blog to get insights sent directly to your inbox.
AI projects in the life sciences industry often follow a familiar arc.
Early pilots generate enthusiasm, and demos captivate the entire C-suite. But a different reality sets in when it’s time to deploy these solutions. Data access, infrastructure gaps, and compliance requirements slowly grind progress to a halt.
We’ve seen this pattern repeat time and again while supporting life sciences organizations through AI delivery. Unlike what most executives believe, the issue isn’t that the models fail or aren’t aligned to strategic goals. It’s that most organizations aren’t set up to operate AI at scale, especially in highly regulated environments.
However, when AI does make it past this barrier, the returns in the life sciences industry are outsized. It is one of the leading industries where AI’s ROI can be easily quantified with faster clinical trials, stronger R&D productivity, and more reliable patient outcomes.
The goal of this playbook is to help your team move past the common friction points in AI deployment. We focus less on algorithms and more on specific decisions that often make or break AI implementation: choosing the right use cases, running pilots with clear success criteria, integrating AI into daily workflows, and putting the right foundations in place.
Where should life sciences executives invest to maximize ROI from AI?
Four areas in the life sciences value chain are currently witnessing some of the highest returns from AI deployment: accelerating research, improving clinical trials, optimizing manufacturing, and enhancing medical affairs. Each of these phases relies heavily on manual effort and high-stakes decisions, making them especially well-suited for AI-driven improvements in speed, accuracy, and consistency. Our CEO, Sharon Lynch, shares in this article how healthcare and life sciences companies can drive value with AI.
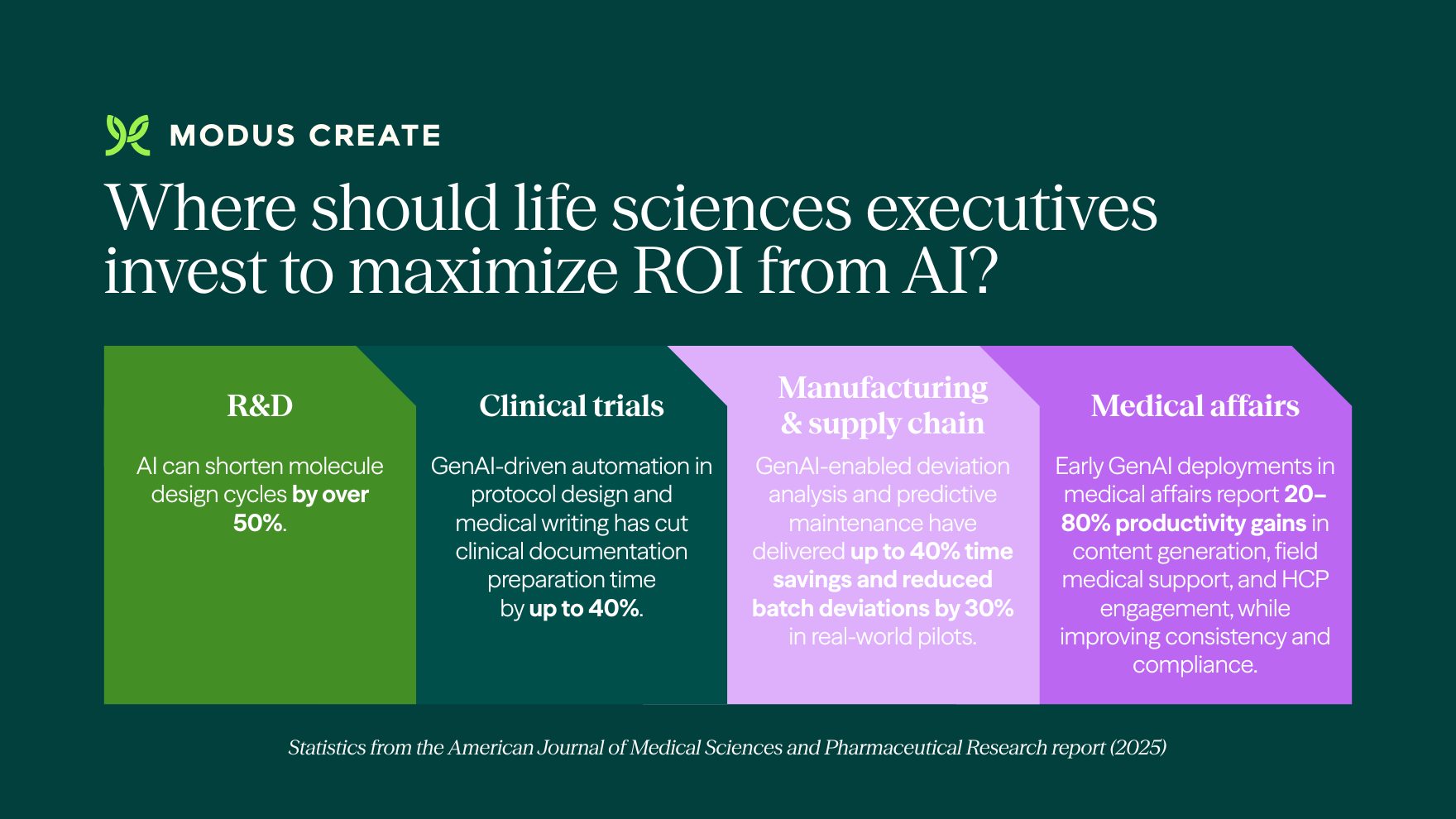
How AI accelerates R&D in life sciences
AI accelerates R&D by narrowing the search space early, allowing teams to focus time and budget on the most promising targets and molecules.
What are the key AI use cases in R&D?
- Target identification: Large-scale omics analysis uncovers novel therapeutic targets and biomarkers, revealing patterns invisible to manual analysis.
- Molecule and protein design: Deep learning and Generative models optimize molecular structures, predict ADME/toxicity profiles, and prioritize candidates for validation, shortening the design-build-test cycle.
- Literature and patent analysis: AI rapidly scans scientific articles, patents, and trial records, extracting insights and generating testable hypotheses.
- In silico simulations: Virtual modeling predicts molecular interactions and clinical outcomes, reducing reliance on costly experiments.
How AI improves clinical trials
AI raises both the scientific precision and operational efficiency of clinical trials by reducing uncertainty in enrollment, surfacing issues earlier, and removing manual bottlenecks in reporting.
What are the key AI use cases in clinical trials?
- Site and patient selection: AI analyzes Electronic Health Records (EHRs) and historical trial data to identify eligible patients faster, improve cohort diversity, and match participants more accurately to study criteria.
- Predictive monitoring: Models anticipate patient dropouts and operational deviations early, allowing teams to intervene before timelines are impacted.
- Documentation automation: Automating protocols, Clinical Study Reports (CSRs), Electronic Case Report Form (eCRFs), and medical coding help create more accurate reports.

How AI transforms manufacturing & supply chain
In manufacturing and logistics, AI shifts operations from reactive quality control to predictive, data-driven execution. This proactive optimization helps life sciences teams improve throughput, reduce deviations, and increase operational stability.
What are the key AI use cases in manufacturing & supply chain?
- Deviation prediction: Real-time monitoring detects anomalies before they impact production and reduces the risk of compliance issues.
- Process optimization: Models continuously refine operating parameters to improve throughput, yield, and consistency across production lines.
- Batch record review: AI automates the review of batch documentation, accelerating release cycles while maintaining audit readiness.
- Predictive maintenance: Equipment and sensor data are used to forecast failures and schedule interventions before downtime occurs.
How AI supports medical affairs & commercial operations
Acting as a scientific copilot, AI helps medical and commercial teams streamline content, insights, and engagement with stakeholders.
What are the key AI use cases in medical affairs & commercial operations?
- Field insights & omnichannel intelligence: Real-world feedback and engagement data are analyzed to inform strategy and optimize operations.
- Explainable medical chatbots: Transparent AI support provides consistent, scientifically validated guidance to HCPs and internal teams.
- Content generation & MLR acceleration: Automated creation and review of medical, legal, and regulatory content ensures speed, consistency, and compliance.

What do life sciences organizations need before launching AI initiatives?
Before launching AI initiatives, life sciences organizations need six core foundations in place: clear strategic ownership, a well-governed data foundation, a scalable and secure infrastructure, strong governance for risk and compliance, AI-ready teams and culture, and operational discipline to validate, deploy, and scale solutions.
How much of this needs to exist upfront depends on the use case. Low-risk pilots can move forward with lighter foundations, while regulated, production-grade AI requires stronger controls from the start. As AI moves from experimentation to enterprise deployment, gaps in readiness become the primary constraint on scale.
Before unpacking the six foundational capabilities, this article offers a practical self-assessment framework with five questions to help executives gauge their organization’s readiness to deploy and scale intelligent AI applications in life sciences.

1. Align artificial intelligence initiatives with business goals from day one
Goal: Align AI initiatives to enterprise priorities and outcomes, with clear executive ownership from day one.
84% of HCLS leaders say following through on strategic elements of developing and launching new products is a barrier to success for their organization — AI in Product Development, Modus Create Research
What to do:
- Define a focused AI strategy that links initiatives to outcomes such as faster molecule discovery, shorter trial timelines, or more efficient review workflows.
- In addition to technical metrics, align each AI effort with at least some business KPIs like trial acceleration, cost reduction, or documentation accuracy.
- Review progress regularly and adjust direction based on real data.
- Secure C-suite sponsorship for each major project so you don’t fall short of resources and support midway through the process.
Tools and frameworks: Strategy mapping frameworks | KPI dashboards | Executive steering committees | Project tracking tools (Jira, Confluence)
2. Build a data foundation for compliant AI
Goal: Create a robust data foundation that is appropriate for the intended AI use cases. Once the foundation is ready, strengthen governance and interoperability as the solution scales.
What to do
- Start with the data that already exists and critical variables relevant to the use case, rather than waiting for perfect enterprise-wide data maturity.
- Apply metadata and interoperability standards (e.g., CDISC for clinical trial data, HL7/FHIR for healthcare data exchange) when required by regulatory scope, reuse needs, or cross-system integration.
- Introduce data catalogs and lineage capabilities incrementally to support traceability, reproducibility, and auditability of AI outputs, especially for GxP-relevant use cases.
- Reduce high-impact data silos selectively to enable collaboration between R&D, clinical, and manufacturing teams where AI value depends on cross-domain data.
- Prepare multi-modal datasets (omics, imaging, EHR, operational data) only when the AI approach requires them, rather than treating them as a prerequisite.
Tools and frameworks: Data catalog platforms | ETL tools | Data governance dashboards | Integration platforms
3. Set up scalable and secure AI infrastructure
Goal: Create an infrastructure that supports AI workloads reliably in production, without introducing security, compliance, or scalability bottlenecks.
What to do:
- Choose a cloud or hybrid setup that balances global scale with data residency and regulatory requirements.
- Ensure availability of GPUs / HPC clusters for large-scale AI training, including omics and imaging data.
- Centralize data in a data lake or warehouse to support analytics and predictive modeling.
- Implement GxP-compliant infrastructure with audit trails, controlled access, and automated compliance checks.
- Build MLOps pipelines for model versioning, monitoring, validation, and automated testing.
Tools and frameworks: AWS (AWS Life Sciences Competency status), Azure, GCP, HPC platforms | MLOps frameworks (Kubeflow, MLflow)
Security is often what slows AI projects down when moving from pilot to production. In this article, Charles Chibueze explains why generative AI introduces new security challenges and how CISOs can address them in practice.

4. Establish governance to manage risk and compliance
Goal: Make AI trustworthy in regulated environments by ensuring decisions can be explained, traced, and defended under audit.
88% of HCLS organizations had their AI deployment slowed down by unexpected regulatory or ethical considerations in the past 12 months — AI in Product Development, Modus Create Research
What to do:
- Define a comprehensive AI risk framework covering ethical, regulatory, cybersecurity, and operational risks.
- Prepare SOPs and validation templates to accelerate regulatory compliance.
- Ensure AI models have explainable outputs and traceable decision paths.
- Implement data security measures: anonymization, encryption, and controlled access aligned with GDPR, HIPAA, FDA, and GxP.
- Continuously monitor for model bias, drift, or deviations from compliance standards.
Tools and frameworks: Governance platforms | Compliance management software | Audit trail tools | AI monitoring frameworks.
In regulated environments, AI programs often slow down at the point where security, compliance, and governance intersect. This article walks through practical ways to mitigate AI security and compliance risks without blocking delivery.

5. Build a culture that encourages artificial intelligence adoption
Goal: Build an AI-ready organization with strong governance, skilled teams, and a culture of rapid learning.
What to do:
- Establish an AI center of excellence to consolidate knowledge, accelerate adoption, and enforce standards.
- Place data scientists and AI specialists directly within functional teams so models reflect real scientific, operational, and regulatory context.
- Encourage experimentation through controlled pilots that allow teams to learn quickly without introducing unnecessary risk.
- Implement change management programs to communicate AI’s role and address concerns.
- Launch continuous training programs in AI, data literacy, and compliance for technical and non-technical staff.
Tools and frameworks: LMS platforms | Collaboration tools (Atlassian tools) | Mentoring programs | Project dashboards | Knowledge bases (Confluence)
In this article, Wesley Fuchter shares why experimentation and learning are often the missing ingredients for sustainable AI adoption.
6. Prepare operations to validate and scale AI
Goal: Ensure AI projects are validated, measurable, and ready for industrialization.
What to do:
- Prioritize use cases based on potential impact, feasibility, regulatory complexity, and resource availability.
- Run tightly scoped POCs with clear success criteria and active involvement from domain experts.
- Define quantitative and qualitative KPIs before each project, including business metrics, operational efficiency, and model accuracy.
- Prepare workflows for model validation, versioning, monitoring, and deployment to ensure smooth scale-up.
- Review performance regularly and refine both models and processes based on real-world usage and feedback.
Tools and frameworks: Project management tools | KPI dashboards | MLOps pipelines | Monitoring platforms
How can life sciences organizations successfully launch AI initiatives?
Life sciences organizations succeed with AI by starting small and deliberate. A single, high-impact use case, clear success metrics, controlled pilots, and defined ownership create the discipline needed to move from experimentation to production. The objective is not AI adoption for its own sake, but AI that teams can trust, operate, validate, and sustain in regulated environments.

Start with a high-value, high-feasibility use case
Many life sciences companies fail by attempting too many initiatives at once. We recommend starting with one AI use case that sits at the intersection of high ROI and low regulatory risk.
Case studies
- Accelerating MLR approvals with GenAI: EVERSANA implemented a GenAI-driven content review and validation workflow that reduced submission errors by 86% and delivered 35% cost savings, significantly shortening MLR approval cycles.
- Automating clinical trial design with AI: Using AI-driven enrollment prediction and optimization, a life sciences organization automated clinical trial country strategy design, improving trial planning speed and generating over $5M in global clinical operations savings.
- Lowering R&D costs: AI adoption in early drug development is associated with 25–50% reductions in preclinical R&D costs, improving resource efficiency across research functions.
For an executive view on prioritizing AI initiatives that deliver real value in Life Sciences, read How healthcare & life sciences companies can drive value.
Run controlled pilots with clear success criteria
Every pilot should be based on evidence, not just technical feasibility. Define success upfront across three dimensions:
- Success metrics: Accuracy, time saved, cost reduction, deviation rate
- Operational KPIs: Process cycle time, model adoption rate, error reduction
- Compliance checkpoints: SOP adherence, traceability, audit-readiness
When regulatory constraints limit access to production data, use synthetic or anonymized datasets to validate model behavior without introducing unnecessary risk.
Remove friction from daily workflows
AI delivers value only when it fits into how teams already work. Integration should remove friction, not add another layer of tooling.
- Connect models directly to core platforms such as LIMS, CTMS, QMS, CRM, and Veeva so insights surface where decisions are made.
- Embed AI outputs into existing workflows to support scientific and operational decisions in R&D and clinical operations.
- Automate handoffs across stages so predictions flow smoothly into validation and action.
Create cross-functional ownership
As AI moves from pilot to day-to-day use, success increasingly depends on clear ownership across functions, not just technical performance.
In life sciences, AI initiatives often stall after early wins when accountability for model decisions, validation, monitoring, and evolution is unclear. Without defined ownership, models become disconnected from business processes, regulatory expectations, and long-term value delivery.
To successfully launch and sustain AI initiatives, organizations should involve from the start:
- Domain experts (R&D scientists, clinicians, QA, medical writers) to ensure AI outputs remain scientifically sound and operationally relevant.
- Data and AI teams to own model development, performance monitoring, and retraining.
- IT and Security to support scalable infrastructure, secure access, and system integration.
- Compliance and QA to embed GxP and regulatory considerations into AI-enabled processes early.
- Business leaders to drive adoption, prioritize AI use cases, and track realized value.
Assign explicit accountability for each stage of the AI lifecycle. Be clear on who reviews outputs, who monitors drift, and who decides when models need to change. Clear ownership is what turns isolated successes into scalable, compliant capabilities.
Scale AI models with MLOps
MLOps is what turns AI from a one-off build into a system that can be run, reviewed, and trusted over time. It provides the structure needed to manage models across their full lifecycle, from development to regulated production use.
- Versioning: Track changes to datasets, features, models, and configurations to ensure full traceability.
- Reproducibility: Ensure models can be reliably recreated across environments and time, a baseline requirement for validation and audit readiness.
- Validation: Continuously assess model performance, robustness, and limitations against predefined success criteria.
- Explainability: Provide transparent and interpretable model outputs to support scientific review, operational trust, and regulatory scrutiny.
- Documentation: Maintain structured documentation covering model intent, data sources, assumptions, validation results, and known limitations.
- Monitoring: Detect model drift, data shifts, performance degradation, or anomalies in real-world usage.
- Security and compliance: Enforce encryption, access control, audit trails, and GxP-aligned controls throughout the AI lifecycle.
With MLOps in place, AI pilots can be promoted into enterprise-grade capabilities that are reliable, compliant, and ready to scale across R&D, clinical operations, and manufacturing.
Build continuous improvement loops
AI impact compounds when models adapt and improve over time:
- Monitor model performance, explainability, and predictive accuracy
- Collect end-user feedback and workflow insights
- Retrain on new data and updated clinical or manufacturing datasets
- Expand AI capabilities to adjacent use cases across life sciences
Continuous improvement turns AI from a one-time deployment into a durable capability that delivers sustained ROI and supports long-term adoption across the life sciences value chain.
How to measure the impact of AI in life sciences?
In our survey of 500+ product and technology leaders, 90% reported increased pressure to prove ROI from AI. For the C-suite, experimentation alone no longer cuts it. The conversation has shifted from what’s possible to what’s delivering measurable commercial impact.
That shift makes KPI selection critical. The metrics you track will determine whether AI is seen as a strategic capability or an expensive experiment. In life sciences, the most effective AI programs measure impact across four dimensions: business outcomes, operational efficiency, data/model performance, and adoption. Together, these indicators show whether AI initiatives are delivering real outcomes, operating reliably in regulated environments, and being used in day-to-day research, clinical, manufacturing, and medical workflows.
For a deeper look at how executives evaluate the ROI of AI-assisted product development and decide which initiatives deserve continued investment, this article breaks down the metrics that matter in practice.
Popular KPIs for AI in life sciences
| KPI categories | Example metrics | Insights |
|---|---|---|
| Business | Time-to-market reduction, cost savings, revenue impact | Link each AI initiative to at least one core business KPI to prioritize high-ROI projects. |
| Operational | Process cycle time, deviation reduction, trial patient dropout | Integrate operational KPIs into executive dashboards for a real-time view of AI’s impact. |
| Data & AI | Model accuracy, drift detection, data quality | Measuring improvements in data quality pre- and post-AI implementation is often overlooked but strongly reflects project maturity. |
| Adoption & Culture | Training completion, user satisfaction, cross-functional collaboration | Combine qualitative and quantitative adoption metrics to detect early resistance to AI deployment. |
Track AI impact with a centralized dashboard
To make KPI tracking operational, use centralized dashboards that bring business, operational, technical, and adoption metrics into a single view.
4 best practices
- Create a single source of truth for AI KPIs accessible to executives, QA, and AI program leads.
- Update dashboards monthly or quarterly, depending on regulatory exposure and operational cadence.
- Configure alerts for critical deviations, model drift, or compliance-related risks to enable early intervention.
- Use KPI trends (not just point-in-time values) to adjust priorities and guide investment decisions.

How to avoid common mistakes in AI adoption in life sciences?
AI can deliver outsized value in life sciences, but only when it’s supported by the right strategy, processes, and ownership. Even strong models fall short when they’re deployed into misaligned organizations. Based on our delivery experience, these are the most common pitfalls we see pharma and biotech companies run into when launching AI initiatives, and how you can avoid them.
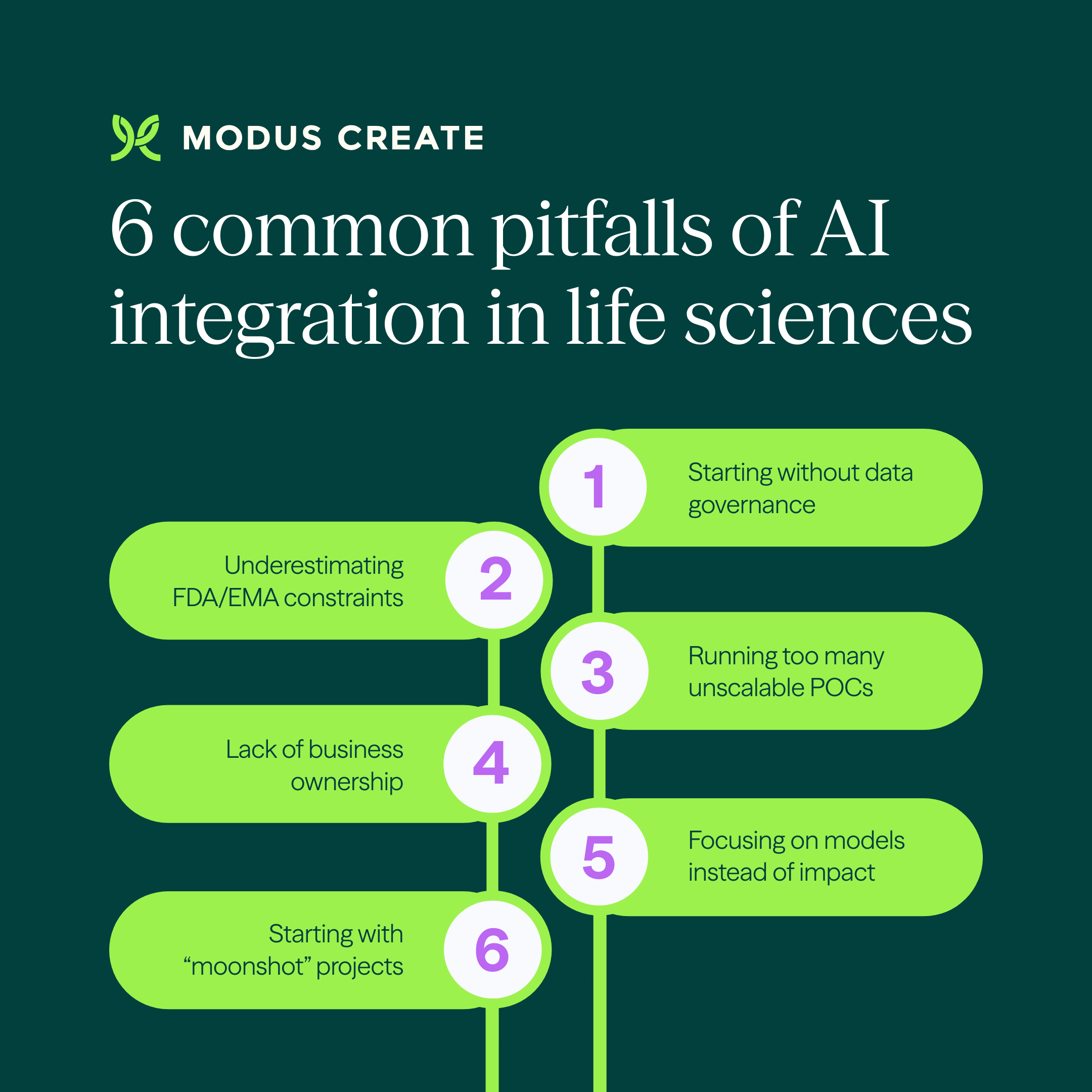
Starting without data governance
AI depends on high-quality, well-governed data. Many organizations dive into projects without clear frameworks for ownership, standards, or integration, resulting in fragmented datasets, inconsistent formats, and unreliable insights.
How to avoid:
- Establish a data governance framework before starting AI projects.
- Define data owners, set standards (CDISC, HL7/FHIR), and ensure proper metadata and lineage tracking.
- Implement data quality monitoring and centralized catalogs to maintain consistency and accessibility.
Underestimating FDA/EMA constraints
AI in clinical trials, manufacturing, or patient care must meet strict regulatory requirements. Teams often overlook the documentation, explainability, and validation needed for FDA or EMA audits.
How to avoid:
- Integrate regulatory requirements from day one.
- Ensure GxP compliance, traceability, and audit-ready documentation for every model.
- Involve QA and compliance experts early in model design to anticipate challenges and streamline approvals.
Running too many unscalable POCs
Launching multiple proofs-of-concept without a clear scaling plan can waste resources. POCs may show potential but fail if models cannot be industrialized.
How to avoid:
- Focus on 1–2 high-value use cases aligned with business priorities.
- Define measurable KPIs for each POC and validate results before scaling.
- Build MLOps pipelines and industrialization workflows in parallel to ensure a smooth transition to production.
Lack of business ownership
AI initiatives led solely by data scientists or IT teams often fail to generate a measurable impact. Without executive sponsorship and business alignment, AI remains a “proof” rather than a solution.
How to avoid:
- Embed business owners throughout the AI lifecycle.
- Align AI initiatives with strategic goals and clearly communicate expected business outcomes.
- Encourage cross-functional collaboration between AI teams and operational units.
Focusing on models instead of impact
A technically perfect model is meaningless if it doesn’t solve a pressing business problem or improve patient outcomes. Teams can get lost in model accuracy, novelty, or AI buzzwords.
How to avoid:
- Prioritize impact-driven metrics: time-to-market, trial success, operational efficiency, cost savings.
- Measure enterprise KPIs alongside technical KPIs to evaluate true value.
- Avoid “perfectionism” in modeling; focus on deployable, actionable insights.
Starting with a “moonshot” project
Ambitious projects are tempting but risky if the organization isn’t prepared. Moonshots can consume resources and fail to deliver quick wins, reducing momentum and stakeholder trust.
How to avoid:
- Begin with achievable, high-ROI initiatives that demonstrate measurable value.
- Use early wins to build expertise, trust, and executive confidence.
- Gradually scale to larger, transformative projects once the organization is ready.
From AI experimentation to execution at scale
AI in life sciences has entered a new phase: from proof of concept to accountable execution. Success now depends on operating it within regulatory, data, and operational constraints. The organizations that succeed are not the ones with the most ambitious pilots, but those that build the discipline to select the right use cases, put the right foundations in place, and operate AI with the same rigor as any other regulated system.
If these challenges resonate, you’re not alone. Many of the patterns outlined in this playbook come directly from real-world delivery. Modus Create works alongside life sciences teams to help move AI from pilot to production: AI strategy, data foundations, engineering, and governance so AI initiatives can be deployed, trusted, and scaled over time.
Frequently Asked Questions

Modus Create is a digital product engineering partner for forward-thinking businesses. Our global teams work side-by-side with clients to design, build, and scale custom solutions that achieve real results and lasting change.
Related Posts
Discover more insights from our blog.


